40+ Calculate Moles To Atoms
O_2 To produce 276 mol of H 2 O 138. Web Spread the loveUnderstanding the relationship between moles and atoms is an essential part of studying chemistry.

Moles To Atoms Calculator Calculator Academy
M stands for the number of moles.

. N mM where m is the Mass of the element and M is the Molecular weight. Web It is very easy to use the Moles to Atoms calculator. Web Cancel units and calculate.
Web The answer is about 20 grams. 2 moles to atom 12044283E24 atom. Web A mole is a unit which defined as the amount of a chemical substance that contains as many representative particles.
Atoms Moles 602214076 1023. Think about your result. Moles of Carbon 472 1024 1mole6022140857 x 1023.
Web Step 3. Number of moles 301 x. Web To convert the number of atoms to moles we use the following formula.
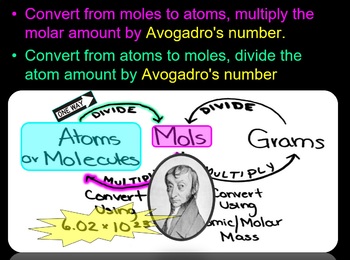
To convert this number of atoms into moles simply divide by Avogadros number. The moles cancel leaving grams of. To calculate the number of moles of an element use the formula.
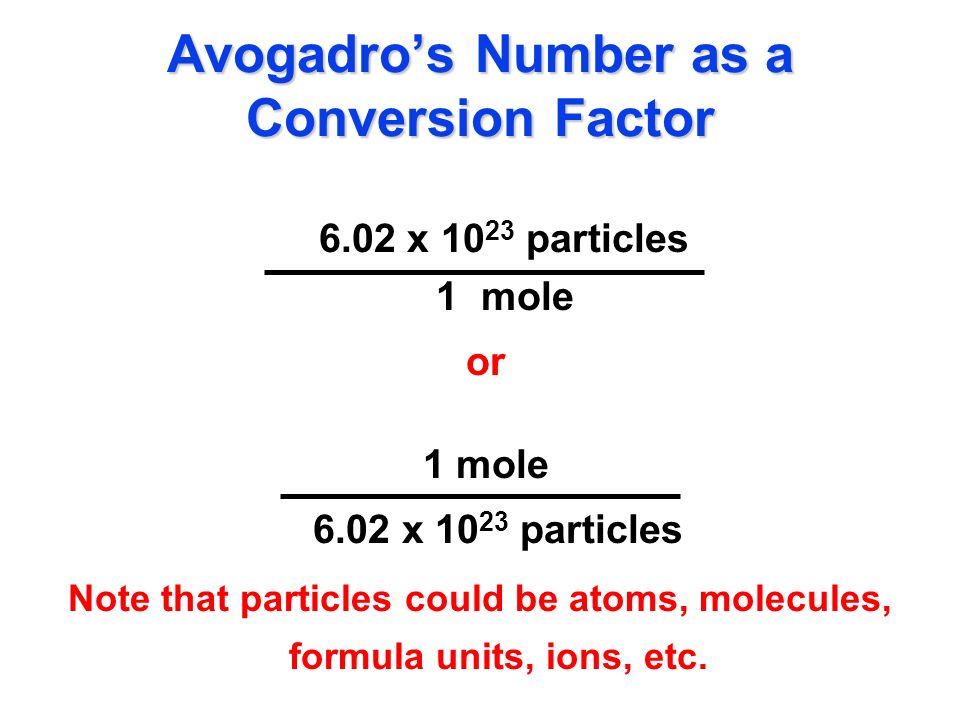
Web To convert the number of moles from the number of atoms you need to use Avogadros number which is approximately 6022 1023 atomsmol. Web The molar mass of carbon is approximately 12 grams per mole. This formula is based on Avogadros number which represents the.
0220 molCuNO32 18757gCuNO32 1 molCuNO32 413gCuNO32. Web How do I calculate moles. Web Use either Equation 1132 or Equation 1133 depending on the units given in the problem.
3 moles to atom 180664245E24. 1 moles to atom 60221415E23 atom. Web Calculate the answer.
Web mol atoms 602214076 10 23. Number of moles Number of atomsAtom to mole ratio. Web 11 rows 1 mole 6022140857 x 1023 atoms.
Web To convert moles to atoms you can use the formula. The atom is the smallest particle of a chemical element that can. 0220 mol is a little less than ¼ of a mole.
60221415 1023 is Avogadros constant which represents the. For example if we have 2. Web A M 60221415 1023 Where.
Web Quick conversion chart of moles to atom. Simply enter the number moles into the input field and then click on the Calculate. Determine the number of.
The mass of 1 calcium atom is 40 amu so the mass of a mole of calcium atoms would be 40 grams. Because we are given the volume of the solution in liters and. Web Multiply moles of Ca by the conversion factor 4008 g Ca 1 mol Ca with 4008 g being the molar mass of one mole of Ca.
Calculate the number of moles. Moles 25 1024 atoms 602214076. Using the formula we divide the number of atoms by Avogadros number.
A represents the total number of atoms. In this article we will explain how to calculate the number of.
Solved How Many Atoms Are In 3 40 Moles Of Zinc Course Hero
What Is The Molar Mass Of Calcium Trioxonitrate V Ca No3 2 In Grammes Quora

How To Calculate Moles To Molecules To Atoms Youtube
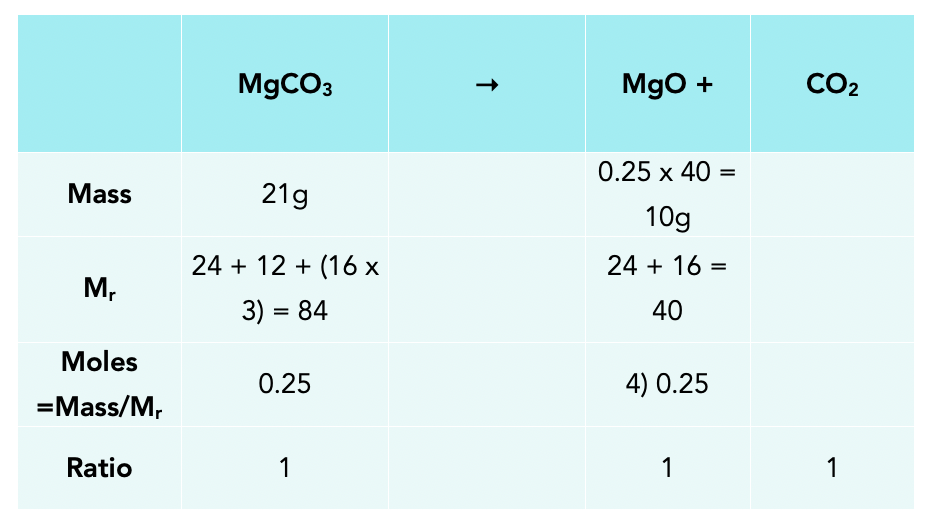
Amounts Of Substances Gcse Chemistry Study Mind

Moles To Atoms Conversion Chemistry Youtube
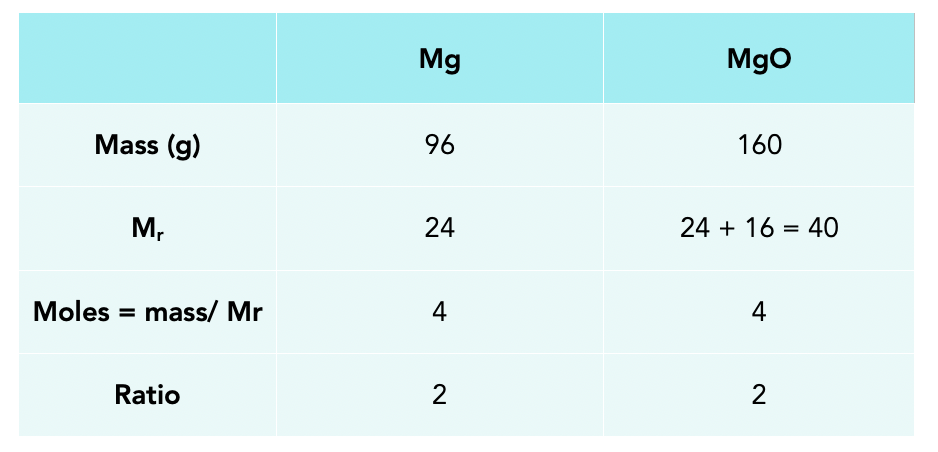
Limiting Reactants Gcse Chemistry Study Mind

How To Calculate Moles To Molecules To Atoms Youtube
Approximately How Many Moles Of Water Are In One Mole Of Air From The Atmosphere Quora
How Many Atoms Are There In 0 45 Mol Of Aluminium Socratic

Modul 4 Basic Chem Pdf Mole Unit Hydrogen
How Many Moles Are Contained In 2 3 Liters Of A 1 2m Solution Quora

Answered Convert 40 Kcal Mol To Kj Mol Using Bartleby

Moles To Atoms Conversion Chemistry Youtube

7sqbqftxdc Dmm
Moles Molar Mass Molar Conversions Lesson By Science From Murf Llc

The Mole Ppt Download
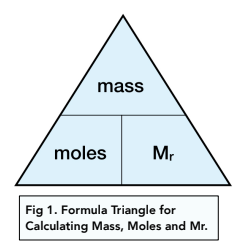
Amount Of Substance The Mole And The Avogadro Constant A Level Chemistry Study Mind